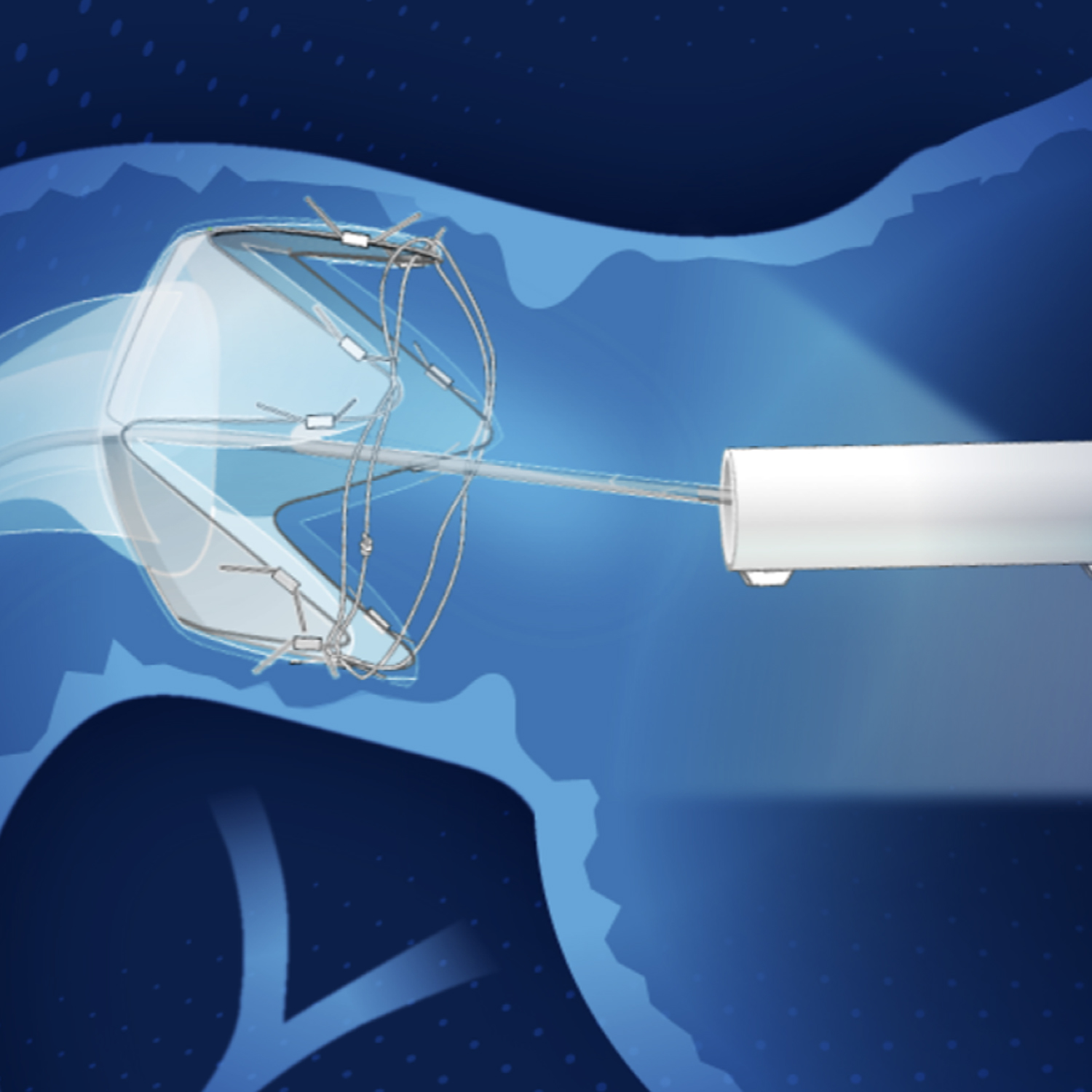
RESET® is a thin, flexible 60 cm sleeve that is endoscopically implanted within the small intestine by a trained physician. The implant secures itself with an anchoring mechanism at the upper portion of the small intestine. Placement is performed as an outpatient procedure that does not require incisions.
Once in place, RESET® conforms to the shape and movement of the intestine and may begin to work immediately by creating a physical barrier between receptors in the intestinal wall and food that has been shown to directly affect key hormone levels.

Clinical studies have shown that RESET® creates changes in gut hormones that mimic mechanisms similar to Roux-en-Y gastric bypass (RYGB)1,2,3 and improves cardiovascular risk biomarkers4 through positive clinical outcomes. Clinical data also suggests RESET® improves glycemic control and reduces weight in patients with Obesity5.
The most commonly reported complications of RESET® are gastrointestinal in nature. They include nausea, vomiting, and upper abdominal pain of mild to moderate severity and are mostly prevalent in the early days and weeks following RESET® placement. Uncommon risks include liver abscess, device-related bleeding, device migration, pancreatitis, or other infections, all of which can be resolved with the endoscopic or surgical removal of the device. As with all endoscopic and/or implant procedures, serious injury or death can occur6.
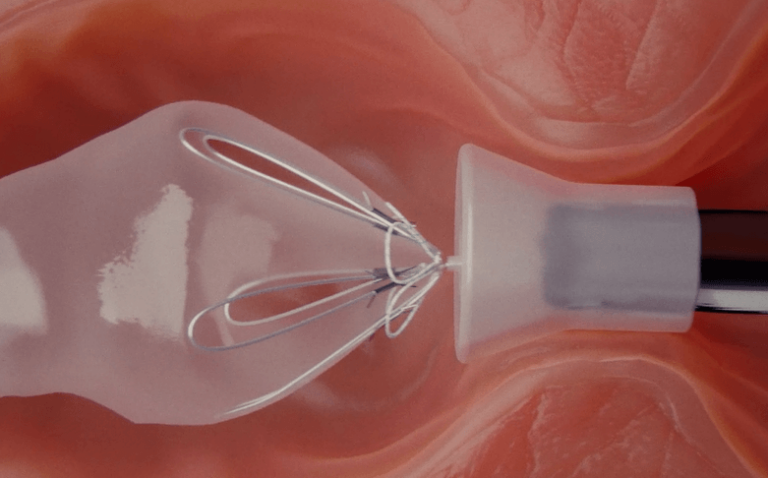
Treatment with RESET® lasts up to 9 months.
Removal of the RESET® device is conducted through an endoscopic outpatient procedure under general anaesthesia.
1 Ruban, A., et al., Endoscopic Interventions in the Treatment of Obesity and Diabetes. Digestive Diseases and Sciences, 2018. 63(7): p. 1694-1705.
2 Ruban, A., H. Ashrafian, and J.P. Teare, The EndoBarrier: Duodenal-jejunal bypass liner for diabetes and weight loss. Gastroenterology Research and Practice, 2018. 2018.
3 Ruban, A., et al., A duodenal sleeve bypass device added to intensive medical therapy for obesity with type 2 diabetes: a RCT. Efficacy and Mechanism Evaluation, 2020
4 Roehlen, N., et al., Duodenal-Jejunal Bypass Liner (DJBL) Improves Cardiovascular Risk Biomarkers and Predicted 4-Year Risk of Major CV Events in Patients with Type 2 Diabetes and Metabolic Syndrome. Obesity Surgery, 2020. 30(4): p. 1200-1210.
5 De Moura, E.G.H., et al., Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diabetes Technology and Therapeutics, 2012. 14(2): p. 183-189
6 Betzel, B., J.P.H. Drenth, and P.D. Siersema, Adverse Events of the Duodenal-Jejunal Bypass Liner: a Systematic Review. Obesity Surgery, 2018. 28(11): p. 3669-3677.